How to Determine Which Lewis Structure Is Best
Try to satisfy the octets of the atoms bydistributing the remaining valence electrons as nonbondingelectrons. Bromine has 7 valence electrons and.
Draw the Lewis Structure.
. Once you have drawn a good Lewis structure your instructor will build the model. Need help with chemistry. The SCN- Lewis structure is a good structure to help you understand.
This is called the octet rule Count all the valence electrons from the atoms involved in your structure. With these two questions we can determine whether this molecule is a lewis base or acid or you can always draw a Lewis Dot structure. Find the Number of Electrons Needed to Make the Atoms Happy.
Sometimes we can write more than one Lewis structure for a particular ion or molecule. Atoms in general dont like charges so having no charge is better. The lewis structure with formal charges closest to 0 is best in the sense that makes a bigger contribution to the resonance hybrid than the other two resonance structures as is stated on page 73 of the course reader.
How to Draw a Lewis Structure Step 1. The best structure has zero formal charges or the smallest possible formal charges on all atoms. Be the first to share what you think.
To draw a Lewis structure the number of valence electrons on each atom in the compound must be determined. When that happens we usually assign formal charges to the bonded atoms to help determine the correct Lewis structure. To generate the Lewis dot structure you have to follow the given steps.
The best lewis structure is B Lets have a look at the rules how we set up lewis structures most atoms want to have 8 electrons or 2 in the outer shell the valence shell. Yes when you are drawing lewis structures you should be evaluating your structure to determine if it is the most stable version. Lewis dot structures can be drawn to show the valence electrons that surround an atom itself.
The resonance structure with a complete octet is. In this step add up the total number of valence electrons from all. Calculate formal charges on all atoms in each structure.
Use two valence electrons to form each bond inthe skeleton structure. This type of Lewis dot structure is represented by an atomic symbol and a series of dots. Calculating the formal charge is also a good tool.
In the input field enter the required values or functions. The response to this question depends on how you interpret the term best As described on page 73 of the course reading the lewis structure with formal charges closest to 0 is best in the sense that it makes a greater contribution to the resonance mixture than the other two resonance structures. When answering questions that involve resonance structures it is important to take into account all of the resonance structures and not only the.
Note that some of the molecules may have more than one central atom. Determine the total number of valenceelectrons. Find the required count of electrons needed to make the atoms complete.
Following the rules for drawing lewis structures should help guide you in determining this. A lot of the time it is possible to draw more than on. Thats it Now your window will display the Final Output of your Input.
Applying Formal Charges to determine best Lewis structures These four species will be explored. Write the skeleton structure of the molecule. To determine the formal charge for an atom we usually follow these rules.
The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. Begin drawing the Lewis dot structure of the molecule. In this video I explain the 3 rules that help us choose the correct Lewis structure for any molecule.
For output press the Submit or Solve button. Determine the best Lewis structure of the following molecules. Assign all lone pairs of electrons to the atom on which we find them.
An atom is considered happy when its outer. Non-valence electrons are not represented when drawing the Lewis structures. What is the best Lewis structure.
A step-by-step explanation of how to draw the BrO4- Lewis Dot StructureNote from the standpoint of formal charge it would be correct to have three of the O. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. In this step add the total count of valence electrons from all the atoms in a bit.
Lewis-dot structure It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. If there are charges in the structure the best Lewis structure places negative charges on the most electronegative atoms and positive charges on the most electropositive atoms. The total number of valence electrons in the entire compound is equal to the sum of the valence electrons of each atom in the compound.
Name the geometry and associated bond angle observed around each central atom. Its essential for predicting molecular geometry molecule polarity and reactivity in a compound. So Al is the central atom which has 3 valence electrons and there are no unbonded electrons as one of each is bonded to each Br however the valence shell of the central atom is not full therefore this.
A step-by-step explanation of how to draw the SCN- Lewis Structure Thiocyanate Ion. CH3N CO2 N20 Cl20 10. For the species listed above write two valid and likely Lewis structures.
The electrons are represented by dot. More Online Free Calculator. The given molecule is perbromate ion.
Created Oct 4 2021. Drawing a Lewis Structure. Sometimes it is impossible to avoid charges so if both resonance structures are charged then the octet rule needs to be considered.
Using Lewis Dot Structures to Show Valence Electrons. Information about different topics. Find the total count of valence electrons to molecules.
You may need a periodic table for this. Find the Total Number of Valence Electrons. Have them verified by your professor before proceeding to the next step.
Determine the Number. Lorain County Community College General Chemistry I CHMY 171 Using formal charge to determine the best Lewis structure. Follow the below steps to get output of Lewis Structure Generator.
To my knowledge there is not a way to know how many reasonable resonance structures. After then define the number of bonds in the given molecule. Divide this number by 2 to obtain the number of electron pairs.
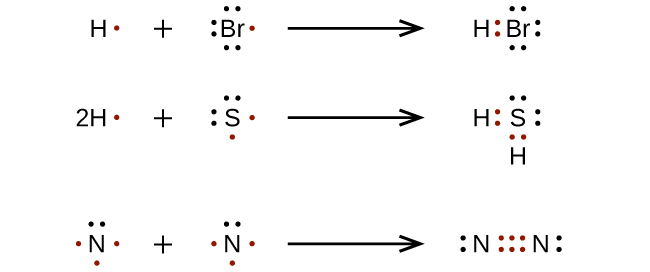
4 3 Drawing Lewis Structures Chemistry Libretexts

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube
Comments
Post a Comment